- Home
- Types & Treatments
- CAR T-Cell Therapy
CAR T-Cell Therapy
The University of Kansas Cancer Center, the region’s only National Cancer Institute-designated comprehensive cancer center, is among the world’s first providers of FDA-approved CAR T-cell therapy and one of only a few centers to offer all FDA-approved CAR T-cell treatments and several clinical trials. This precision cancer therapy offers new potential to cure cancer and save lives.
What is CAR T-cell therapy?
Chimeric antigen receptor (CAR) T-cell therapy uses reengineered versions of a person's own cells to find and fight cancer cells. T cells (integral to the immune system) are extracted from the patient, genetically modified, and returned to the patient's bloodstream to seek and destroy remaining cancer cells.
Who can have CAR T-cell therapy?
Currently, CAR T-cell therapy is FDA-approved for the treatment of:
- Children and young adults up to age 25 with relapsed or acute lymphoblastic leukemia (ALL)
- Adults with refractory, aggressive non-Hodgkin lymphoma
- Adults with relapsed or refractory B-cell acute lymphoblastic leukemia
- Adults with relapsed or refractory mantle cell lymphoma
- Adults with relapsed or refractory multiple myeloma
FDA-approved CAR T-cell therapy is used only after other more traditional therapies have been tried and failed to work. It is being studied as an effective treatment option for other types of cancers and may be available to some patients through clinical trials. For example, research and clinical trials are in process to explore the effectiveness of CAR T-cell therapy to treat breast, colon and lung cancer as well as additional blood cancers. Learn more about clinical trial options that could be right for you.
How does CAR T-cell therapy work?
CAR T-cell therapy uses reengineered versions of your own immune cells to find cancer cells and defeat them. These cells, known as T cells, are the backbone of the immune system and normally lead the charge in killing cancer and other harmful cells.
CAR T-cell therapy separates T cells from your blood and, in a lab, genetically engineers them to include synthetic receptors called chimeric antigen receptors (CARs). These receptors actively search out cancer cells, destroying them as they would a simple pathogen.
You’ll hear a lot of new vocabulary as you become familiar with CAR T-cell therapy. A few of the most commonly used terms include:
- Immunotherapy: A form of treatment that uses your immune system to fight disease.
- Chimeric antigen receptor (CAR) T-cell therapy: A type of immunotherapy that involves re-engineering your T cells to recognize and attack cancer cells.
- Engineered cell therapy: Treatment using genetically modified T cells.
- Infusion: The administration of fluids through a vein into your bloodstream.
- Leukapheresis: The process of removing blood to isolate and collect white blood cells.
Bench to Bedside: Immunotherapy
Dr. Roy Jensen: Welcome to the first episode of Bench-to-Bedside, a weekly series of live conversations about recent advances in cancer, from the research bench to treatment at the patient's bedside. I'm Dr. Roy Jensen, Director of The University of Kansas Cancer Center, and with me is Dr. Joseph McGuirk, who is our Director of The Division of Hematologic Malignancies and Cellular Therapeutics. Together, we're gonna help you better understand CAR T therapy, a type of immunotherapy that is changing the landscape of cancer treatment. What is immunotherapy and how does it work?
Dr. Joseph McGuirk: Immunotherapy is an exciting and very promising area in medicine, and it leverages our increased understanding of how the immune system works, so that we can manipulate that immune system to improve our cancer patients with medical illness. In cancer medicine we call this immuno-oncology.
Dr. Roy Jensen: How is immunotherapy different from cellular therapy?
Dr. Joseph McGuirk.: Well cellular therapy actually is using entire cells of the immune system. The most important of which are cells called T-cells, and it falls under the umbrella, the general umbrella, of immunotherapy. There are other types of immunotherapy that are being leveraged to treat cancer patients as well, for example cancer vaccines, molecules such as antibodies that block interactions between cancer cells and those T-cells' part of the immune system, to prevent them from down-regulating the immune system's response to them in and eradicating them.
Dr. Roy Jensen: You hear a lot of buzz these days about this term CAR T, why is that so exciting?
Dr. Joseph McGuirk: CAR T-cell, Chimeric antigen receptor modified T-cells, which is a mouthful, is an extraordinarily exciting area, because with this, cells are taken out, re-engineered in the laboratory to do the work that they fail to do in the first place, and that is recognize and destroy cancer cells. Re-engineered to do that work again, expanded and infused back into the patients, and we're seeing some truly stunning results in several diseases.
Dr. Roy Jensen: If you're just joining us, we're talking about an exciting medical breakthrough in cancer treatment call CAR T therapy, and Lindsey Leesmann is here in the studio to take your questions. So, remember to share this link with people who might benefit from this discussion. Send us your questions, and remember to share this link and use the hashtag BenchToBedside. How does CAR T therapy work exactly, and which cancers are being treated with this modality?
Dr. Joseph McGuirk: Very important question, so the principal cancers that have been targeted so far with this new technology in manipulating the immune system, have been blood cancers. The CAR T-cells are the patient's own immune system, taking their cells out their blood and isolating these important mediators of the immune system T-cells, and then genetically engineering them to recognize the cancer cells, expanding them, and infusing them back into the patient so they will recognize, attach to the cancer cell, punch a hole in it, and kill the cancer cell. Very exciting, two recent Food and Drug Administration approvals for this therapy and acute lymphoblastic leukemia in children, adolescents, and young adults, and non-Hodgkin's lymphoma in adults, have just come to the forefront.
Dr. Roy Jensen: Are we going to be able to use this type of therapy against solid tumors like breast cancer, colon cancer, and the like?
Dr. Joseph McGuirk: I believe so. There are currently 450 clinical trials in the United States that are exploring further expansion of this CAR T-cell technology, based on the remarkable results in these two diseases that I just mentioned, these blood cancers. Many of those clinical trials do include solid tumors, such as breast cancer, ovarian cancer, lung cancers, and other.
Dr. Roy Jensen: We talk about this being a new therapy, but how long has this therapy been in the development process?
Dr. Joseph McGuirk: This has been in the works for decades, on the shoulders of thousands of men and women in laboratories and scientific laboratories, in translational research efforts throughout our nation. One step after another in understanding in how the immune system works, which is fundamental to this entire process, then experiments that manipulate that immune system to work to the advantage of our patients, and now through clinical trials coming to fruition as a standard therapy for at least two diseases, and more to come.
Dr. Roy Jensen: This is an entirely different approach to treating cancer, could you tell us a little bit about the steps that go into re-engineering these cells?
Dr. Joseph McGuirk: Yes, absolutely. So patients arrive at the cancer center for consideration of such therapy, and the next steps are somewhat dependent on whether they're on a clinical trial, the rigorous criteria for entry in a clinical trial, or whether they're being treated now with standard FDA approved therapeutics. Generally those steps are very much overlapping. It only takes a couple of days to get that sorted out, and make sure that they're an appropriate patient for this therapy and they can tolerate this therapy. They then have blood collected in the laboratory, just like donating blood with a machine, and over a several hour period. That blood is sent to the laboratory for engineering. The T-cells that I mentioned earlier are isolated, they're expanded in the laboratory, and then they're genetically altered to recognize the cancer cells. They're then further expanded to millions of cells. They're frozen, they're sent back to our center generally about 17 days later. Thawed out and infused intravenously into our patient. Those cells sweep through the bloodstream, through the organs, recognize the cancer cells, attach, have a mechanism whereby they punch a hole in the cancer cell and let little Pac-Men enzymes that go in and tear up the cell's DNA, the cancer cell's DNA, so the cell dies.
Dr. Roy Jensen: That's amazing. What is happening with the patient during those 17 days when the cells are being re-engineered?
Dr. Joseph McGuirk: It's a precarious time, a time that we and the patients and their families, of course, worry a great deal, because these patients are most commonly relapsed from prior therapy, and they're resistant to other subsequent therapies. So that's a terrible situation for a patient to be in. Some of these tumors, particularly acute leukemias and lymphomas can grow very rapidly, even in those 17 days while the patient is waiting. There are some things we can do to bridge the patient, to prevent further progression of their disease. Chemotherapy has limited effects in that setting, so we're hopeful that we can keep a hawk's eye out on the patient. They're here in our center, watched and give therapy to hold the fort till we can get those cells back, and get them into the patient.
Dr. Roy Jensen: You've been treating leukemia and lymphoma patients for a long time, could you give us a bit of perspective in terms of the effectiveness of our traditional forms of therapy, versus CAR T therapy?
Dr. Joseph McGuirk: Yes, absolutely. It's really revolutionary, what's happening, and that's not hyperbole. The prime examples are acute lymphoblastic leukemia in children and young adults who relapse and are not responding to subsequent chemotherapy, or who have had a stem cell transplant and relapsed. Their survival time is marked in months, not years, and many of them will succumb to that disease in a matter of weeks. So a terrible situation. Only 6% of those such patients will be surviving it two years afterwards. So, devastating. With CAR T-cell therapy in that very population of patients, 80% to 90% of patients have gone into complete remissions. The majority of those patients with those remissions, are in continued remission beyond six months after being treated. That is unheard of in that population of patients. In adults, in older adults, with non-Hodgkin's lymphoma, the most common type of which is diffuse large cell lymphoma, when they relapse and are resistant to chemotherapy, or have had a stem cell transplant and relapsed, their chance of responding to subsequent therapy is 7%. Their survival at two year is 16%, and half of the patients will be gone by six months. Again, a devastating cancer diagnosis. So in those patients, with CAR T-cell therapy, that very population, 50% of patients are going into complete remissions, and, again, beyond six months, the majority of those patients are staying in complete remissions.
Dr. Roy Jensen: Wow! It's easy to see why this is seen as a revolution. Could you tell us a little bit about the risk of this therapy, 'cause no therapy is without risk.
Dr. Joseph McGuirk: Absolutely, and this therapy is certainly not without risk. There are two general risk categories that these patients have to contend with and confront. One is called cytokine release syndrome, so when we get the flu, those are our T-cells attacking the virus, the virally infected cells, and they release molecules to call in the troops. Those molecules make us have high fevers, can make us have chills and lay in bed for three days. When these CAR T-cells attack the cancer cells, they do the same thing, the call in the troops, but tenfold, and so these patients can develop dramatically high fevers, decreases in blood pressure, become quite ill, and some require intensive care unit stays, and intensive care unit support. The other category of toxicities, is neurological toxicity, which we don't understand well yet. It's an area of great exploration right now. The patients can become mildly confused, have difficulty speaking, or have full-blown seizures and also require intensive care unit stays. So this therapy is not without significant risk. Fortunately, for the overwhelming majority of patients, those toxicities are transient, although there have been some deaths, they've been far and few between, thank goodness. And there have been some rare late sequelae down the line.
Dr. Roy Jensen: Could you tell me a little bit about kind of the nuts and bolts of how a patient accesses these trials. Is there a waiting list? How do you qualify for these types of therapies?
Dr. Joseph McGuirk: A very, very important issue. Now that there are a handful of centers in the United States, including our own, that offer this type of therapy in either the context of a clinical trial, or as standard therapy, as I've describe, for acute lymphoblastic leukemia and non-Hodgkin's lymphoma. Patients, generally, are referred by their physicians from around the nation, and we've actually had patients, as have the other centers in the country, come from around the world, from other nations to our centers for this type of therapy. We move them quickly, because again it's a high-risk population of patients. So, if they're out in California, or they're in Australia, or they're out in the Kansas countryside, we get them in within a day, very quickly. We get them on a plane and get them out here, do a quick workup on those patients to determine whether they are eligible for a clinical trial, or are they eligible to receive the standard therapy with this treatment. Can they tolerate the toxicities that I've just described, or do we have a good chance of getting them safely through this therapy. Once that's been determined in a couple of days, we then go on, as I've described previously, and collect those cells. Then we hold the fort until we can get those cells engineered and back to the patient.
Dr. Roy Jensen: If you're just joining us, we're talking about CAR T therapy, a type of revolutionary therapy to treat cancer. Lindsey do we have any questions from the audience?
Lindsey L.: One question is how long does the qualification process take? You said you get patients in really quickly, but how long before they get in the door to be able to be qualified?
Dr. Roy Jensen: Joel?
Dr. Joseph McGuirk: That's a very important question, and it comes to the critically important point, that it takes a large healthcare team to make this possible. From the financial end, of which can be a substantial challenge for patients and their families, the insurance approval, the coordination of getting them to our center quickly, and the doing the workups very quickly, getting them to the therapeutics. Because, again, this is a highly vulnerable population of patients who have relapsed, so they have active cancer, and substantially so, and they're resistant to other attempts to get them back into remission. So very vulnerable population. We don't have weeks for these patients, we have to move in a matter of days. And again, that takes a complex team. We call that team, in our center, the CAR hub team, and we've published this past September, an article in a journal called Cytotherapy, on how to put such a team together. What are all the parts and pieces that make this go quickly, and smoothly, and safely for our patients.
Dr. Roy Jensen: Those of us at The University of Kansas Cancer Center have already the unique position to offer this therapy to patients, could you go into that a little bit more, about what is it that makes this place a core CAR T center?
Dr. Joseph McGuirk: A couple of very important variables that were critical to our being able to offer these therapies to patients. One, we're a National Cancer Institute designated cancer center, and so that brings a reputation for our center of a commitment to clinical trials, a demonstration that we have the infrastructure to support those clinical trials. Very complex clinical trials, as you can image with this type of therapy, and many other types of cancer therapy. So that's a mainstay pillar of being able to participate in these kind of trials around the nation. And then, secondly, we've had the infrastructure in our stem cell transplant program, and CAR T-cell therapies have been available principally, and I believe exclusively, only in stem cell transplant centers that are approved by a national accrediting body. A lot of rigor goes into that as well. And then a track record through our NCI designation of performance in clinical trial enrollment, in doing those clinical trials well and safely for patients.
Dr. Roy Jensen: About how many places across the United States are now offering CAR T therapy?
Dr. Joseph McGuirk: It depends on whether we look from the clinical trial perspective, or the approved sites for the Food and Drug Administration approved constructs. The former, the clinical trials, many centers around the country are moving in this space, again with very specific teams that are able to do this. That's a minority of even academic centers in the nation, right now. And then in the commercial space, recently approved, we are one of 16 approved sites in the United States to employ those CAR T-cells for patients with lymphomas and leukemias, as I described.
Dr. Roy Jensen: For the FDA approved therapy, obviously patients around the Kansas City region have access to our center, what's the next closest center?
Dr. Joseph McGuirk: Barnes-Jewish Hospital, Washington University in St. Louis, University of Nebraska's been participating in this area, and MD Anderson Cancer Center in Houston.
Dr. Roy Jensen: Is there a waiting list for patients?
Dr. Joseph McGuirk: There is a waiting list, including in our own center, and again that is a challenge, because these are patients with very aggressive diseases who are not responding to therapy, and so moving patients along in a timely manner is absolutely essential. It's a life and death issue for these patients, and so we are, none of us in the United States, the centers that are participating in this area, are shy about calling each other to find out if there's an available slot, if there's not an available slot at our center. We've been fortunate that our infrastructure has supported, and no one has had to wait or to be sent elsewhere to date, and we're hopeful that as we continue to expand our infrastructure, we'll be able to accommodate these patients in a very timely manner.
Dr. Roy Jensen: Great. Lindsey, do you have another question for us?
Lindsey L.: I do. We have a question, do we ever see the 17 day turnaround timeframe decreasing?
Dr. Joseph McGuirk: There are important research efforts underway in laboratories to look at improving that turnaround time. It's actually ... the 17 days is a significant improvement over where we started, was for some of our clinical trials, twice that length. And so many patients, unfortunately, had progressive disease and succumbed with their disease, or were too ill to proceed in the early stages of the clinical trials, including those that we participated in. That timeline has been brought down significantly. It's still, 17 days is a critical 17 days, but I do believe that there are technological improvements that will allow us to shorten that further.
Dr. Roy Jensen: How has the insurance industry approached CAR T therapy? And what are the costs involved here?
Dr. Joseph McGuirk: The costs are very substantial. This is an extraordinarily costly therapy. When we put that in the context of life here saved and productivity in our society, we believe that that is an appropriate balance. However, the upfront costs are very substantial. There's great risk to patients, to institutions, the insurance carriers. So, the insurance carriers to date, in our interactions, have been very responsible, and they've been very supportive. But, boy, they make sure that you've done your homework, and that you're treating the appropriate patients, and patients who have the optimal chance of benefiting from this type of therapy. I think that that is appropriate. When we pull our team together, this is a frontline question that's addressed by part of our healthcare team, the financial arm, to make sure that we're not going to put the patient at undue financial risk, or the institution, and that we'll have healthcare insurance approval. We actually run several processes in parallel, the evaluation of the patient, the communication throughout the team, the preparation to have the cells produced, and the insurance approval. We can't move forward without that insurance approval. But the insurance carriers understand what we're all after here.
Dr. Roy Jensen: Obviously we became involved very early with CAR T therapy trials for the benefit of our patients. How does involvement in these trials benefit the cancer center?
Dr. Joseph McGuirk: It's really critically important. We're very fortunate to enroll patients with non-Hodgkin's lymphoma, to be the first in the nation, and actually the first in the world, in a multicenter multinational trial of CAR T cells for non-Hodgkin's lymphoma. And we have been the leading roller on one of the most important trials in this space. That has brought a lot of attention to our center. Our center already had been involved over many years in many clinical trials, and demonstrating our excellence, which got us to that point. And this further demonstrates to the pharmaceutical industry, to other academic centers, to the National Cancer Institute, that we can contribute, and we can contribute significantly when we're involved in high-priority high-impact studies such as this.
Dr. Roy Jensen: Great. Lindsey, do we have any more questions we want to slip in here?
Lindsey L.: We don't.
Dr. Roy Jensen: All righty. Well, Joel, I just want to congratulate you and your team on an extraordinary amount of work that you've put into making sure that our patients have access to some of the most exciting therapeutic advances that I've ever witnessed in my career in cancer research, and we are truly blessed to have you and all those folks over there, doing such a great job for our patients. Do you have any final thoughts you want to share with folks, all of us?
Lindsey L.: No, thank you. It's the most exciting time of my entire career. I've been a cancer specialist for ... getting up here to 30 years pretty soon. Have always been excited about the prospects of improving lives for our patients, as have you and our other team members, but in no time in my life have I been more optimistic and excited about the developments coming down the line. This imunnotherapeutic approach is just one of a multitude of avenues where we're going after these cancers more successfully, as you know better than anyone.
Dr. Roy Jensen: Well thank you so much for being with us this morning, Joel. If you have any more questions about immunotherapy and cellular therapeutics, go to kucancercenter.org/CAR-T. Next Wednesday at 10:00 a.m., we will look at HPV related cancers and the vaccine that prevents them. In the meantime, share this conversation using the hashtag BenchToBedside with people you know will benefit. See you next week, and thank you for watching.
Benefits and risks of CAR T-cell therapy
CAR T-cell therapy shows remarkable responses in both children and adults for whom other therapies may have stopped working. For many children with acute lymphoblastic leukemia (ALL), CAR T-cell therapy often becomes the only treatment option that works.
As with any complex treatment intended to destroy the most aggressive cancers, side effects may occur. These can include breathing difficulty, fever, chills, confusion, nausea and muscle pain.
As such, it is critically important to get treatment at a highly qualified cancer center with the experience, staff and infrastructure necessary to provide complete care before, during and after the actual administration of CAR T cells.
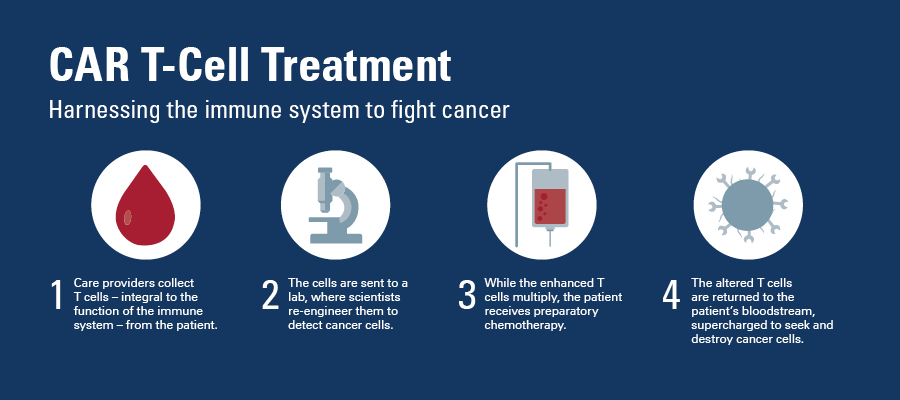
What happens during CAR T-cell therapy?
CAR T-cell therapy uses the body’s own immune system cells to fight the cancer. Here is how it works:
There are currently 7 FDA-approved CAR T-cell therapy treatments: Abecma®, Aucatzyl®, Breyanzi®, Carvykti™, Kymriah®, Tecartus® and Yescarta®. The University of Kansas Cancer Center offers all 7 therapies.
Why choose us
As home to the region’s largest and most experienced blood and marrow transplant (BMT) and cellular therapeutics program, The University of Kansas Cancer Center is uniquely qualified to provide CAR T-cell treatments. Our multidisciplinary care team is highly versed in the specific needs of patients with blood disorders and the care options and risks involved in treating them. We offer the significant infrastructure required to deliver and manage this complex treatment.
Since 1977, our BMT and cellular therapeutics program has provided advanced, compassionate care and performed more than 5,000 transplants and more than 400 CAR T-cell therapy procedures. It represents a powerful foundation on which to advance expertise as immunotherapy increasingly takes its position among frontline cancer treatment options.
We are pleased to provide resources to help inform you as you explore this critical possibility in your cancer care and to earn your confidence as you consider our team and program.
Request your appointment today.
To make an appointment at The University of Kansas Cancer Center, call 913-588-1227.



